On March 13, 2020, Speyside Medical filed a complaint alleging patent infringement by Medtronic’s transcatheter aortic valve replacement (“TAVR”) lines of self-expandable devices that include Medtronic’s CoreValve, Evolut R, Evolut Pro and Evolut Pro+ valves.
Transcatheter Aortic Valve Replacement (TAVR) is a minimally invasive procedure for treating patients who are at risk of undergoing surgical therapy in severe aortic stenosis. Aortic Stenosis (AS) is a condition characterized by the narrowing of the aortic valve due to calcium buildup. This buildup stiffens the thin leaflets of tissue that open and close to regulate blood flow to the aorta making it difficult for the heart to pump blood to and from the body.
TAVR replaces the aortic valve without removing the old, damaged valve. These replacement valves can be broadly classified into self-expandable and balloon-expandable valves. Major players in this space include Medtronic, Edwards Lifesciences, St. Jude Medical (Abbott), Boston Scientific, JenaValve Technology, Symetis and NVT AG.
On March 13, 2020, Speyside Medical filed a complaint alleging patent infringement by Medtronic’s transcatheter aortic valve replacement (“TAVR”) lines of self-expandable devices that include Medtronic’s CoreValve, Evolut R, Evolut Pro and Evolut Pro+ valves. The CoreValve system includes a transcatheter aortic valve (TAV), a delivery catheter, and a loading system. The TAV is an artificial heart valve made of trileaflet porcine pericardial tissue attached to a flexible, self-expanding nickel-titanium (Nitinol) frame for support. The TAV is mounted on the end of the delivery catheter and inserted into the body by direct, transfemoral or transapical approach. The valve is then released from the catheter; expands on its own; anchors to the diseased valve, and functions as a new valve, once in place.
 Source: MaxVal’s Litigation Databank
Source: MaxVal’s Litigation Databank
Speyside Medical’s patents-in-suit (US8377118, US9510941, US10449040, US9445897, & US9603708) relate broadly to devices and methods used in valve replacement surgery and are briefly described below.
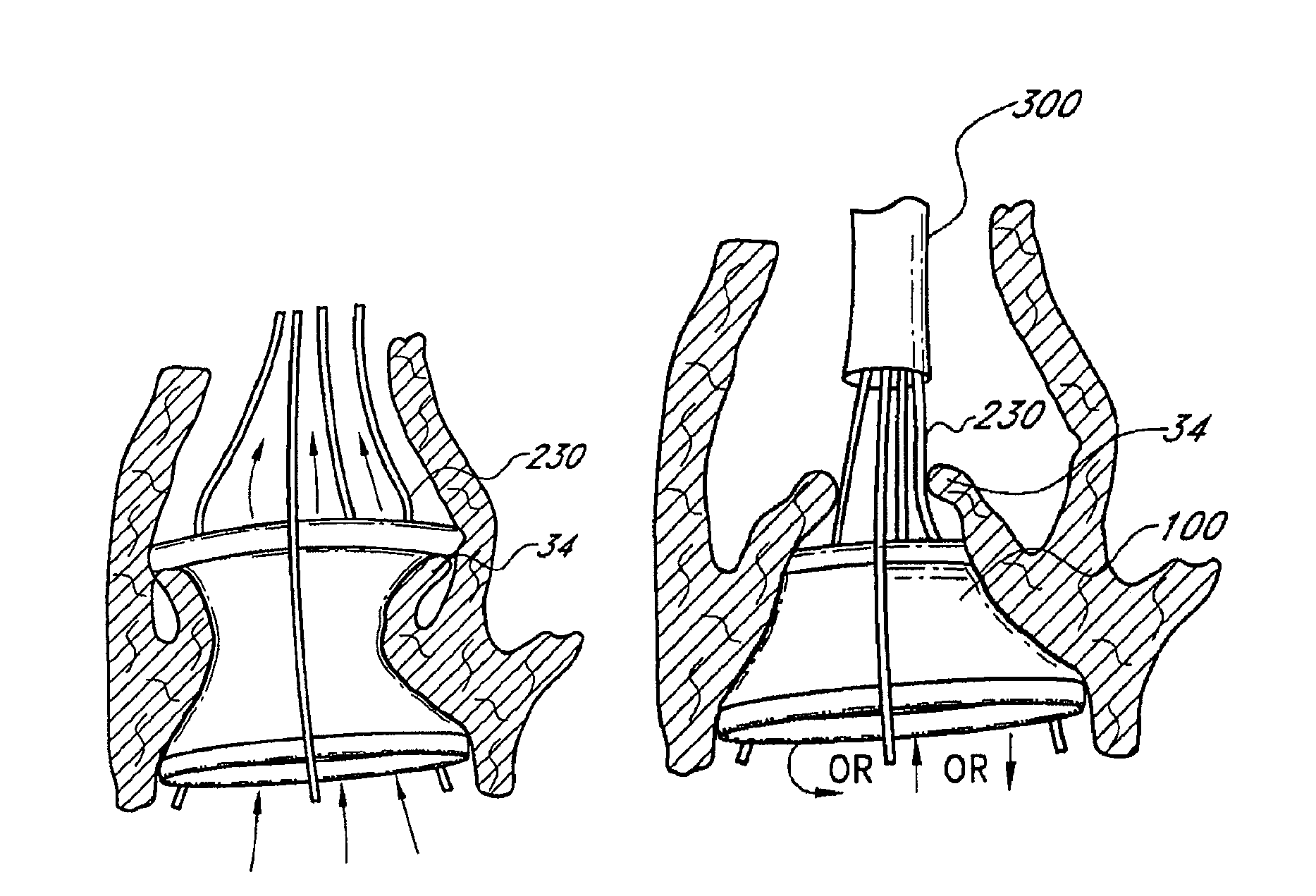
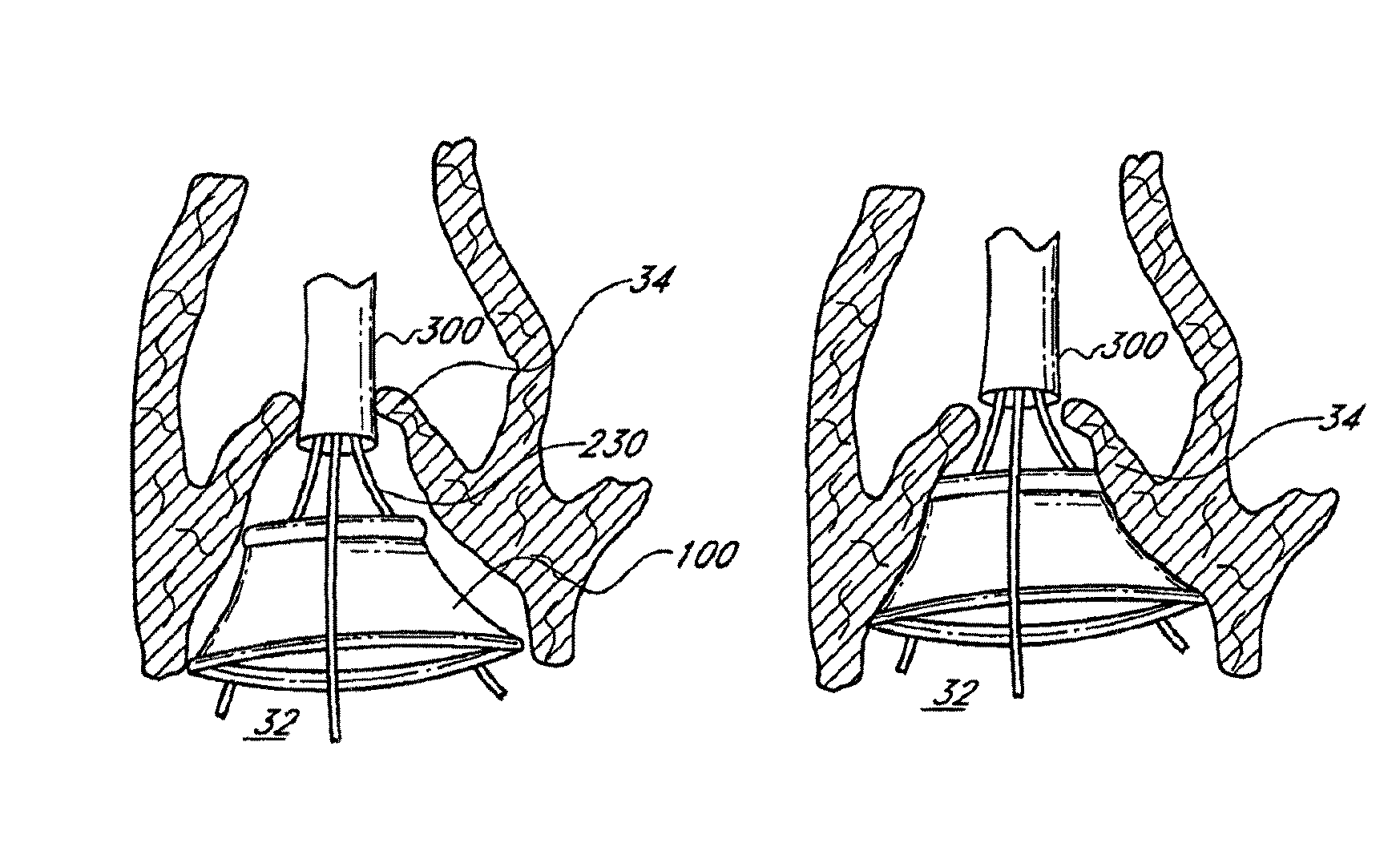
US Patent No. 8,377,118 titled “Unstented heart valve with formed in place support structure”, granted on February 19, 2013, relates to an implantable prosthetic valve with an in situ formable support structure. The valve comprises a first flexible component, a second flexible component, and a rigidity component to retain the valve at the site. The replacement valve is endovascularly delivered to the native aortic heart valve site with an implantable expandable carrier element.
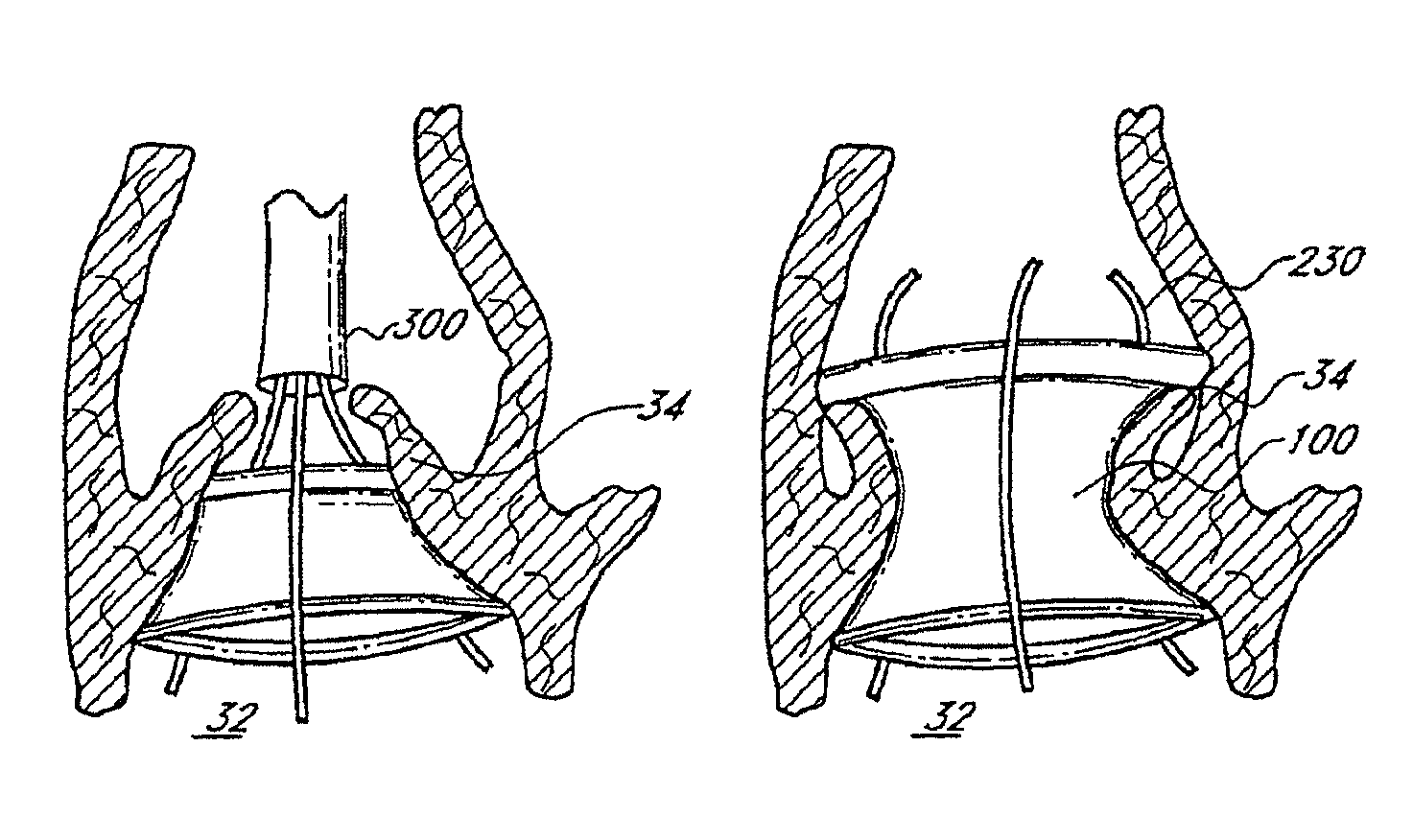
US Patent No. 9,510,941 titled “Method of treating a patient using a retrievable transcatheter prosthetic heart valve”, granted on December 6, 2016, relates to an implantable prosthetic valve with an in situ formable support structure. The valve comprises a first flexible component, a second flexible component, and a rigidity component to retain the valve at the site. The replacement valve is endovascularly delivered to the native aortic heart valve site with an implantable expandable carrier element. A first carrier element with a first replacement valve is expanded, evaluated, collapsed and exchanged with the second carrier element with a second replacement valve.
US Patent No. 10,449,040 titled “Method of treating a patient using a retrievable transcatheter prosthetic heart valve” granted on October 22, 2019, relates to a method for replacing a patient’s native heart valve by delivering a prosthetic valve at the cardiovascular site from a collapsed delivery configuration to an expanded configuration. The method allows reversing the deployment of the prosthetic valve based on performance testing and, if necessary, repositioning or re-deploying the valve.
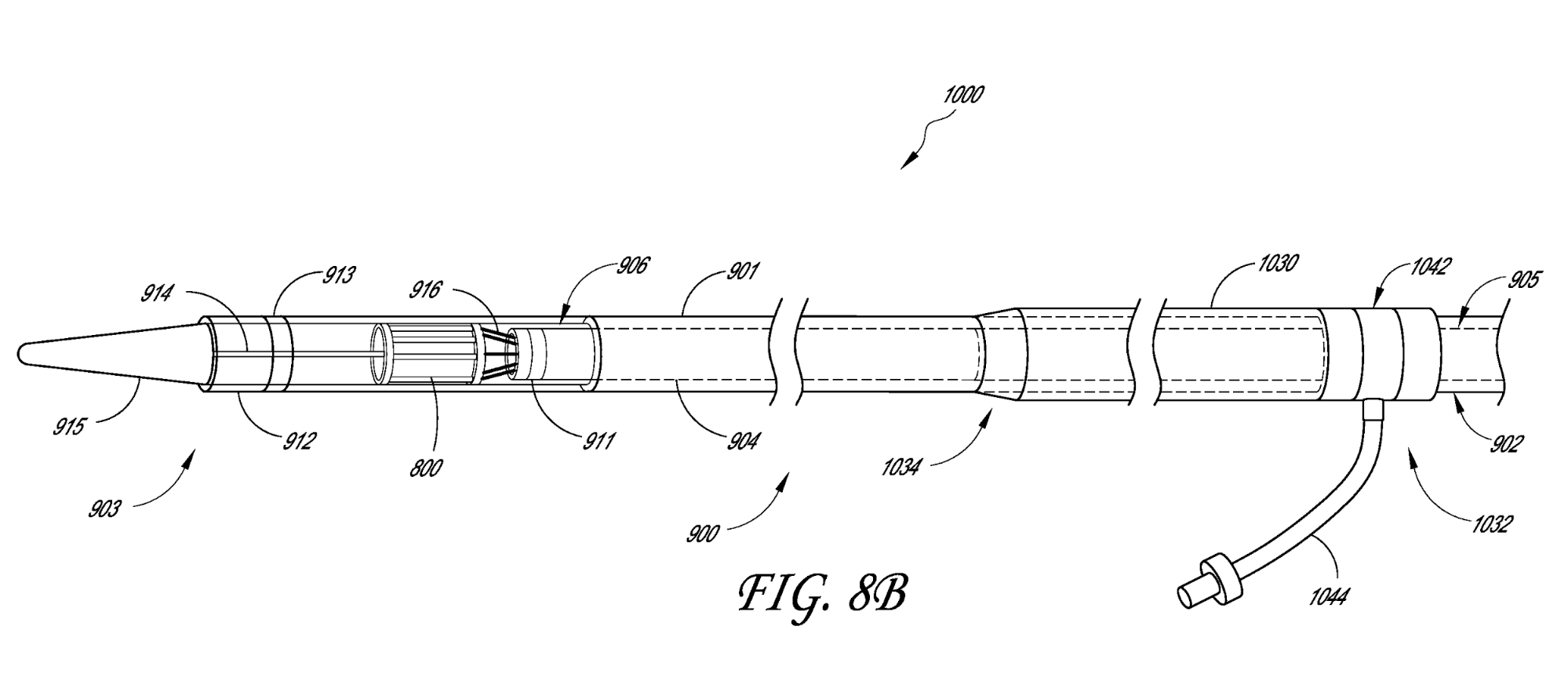
US Patent No. 9,445,897 titled “Prosthetic implant delivery device with introducer catheter” granted on September 20, 2016, relates to a minimally invasive delivery method for deploying a cardiovascular prosthetic implant using an introducer catheter, a delivery catheter having a proximal end and a distal end, and a seal assembly, wherein the outer diameter of the distal end of the delivery catheter is greater than the inner diameter of the distal end of the introducer catheter.
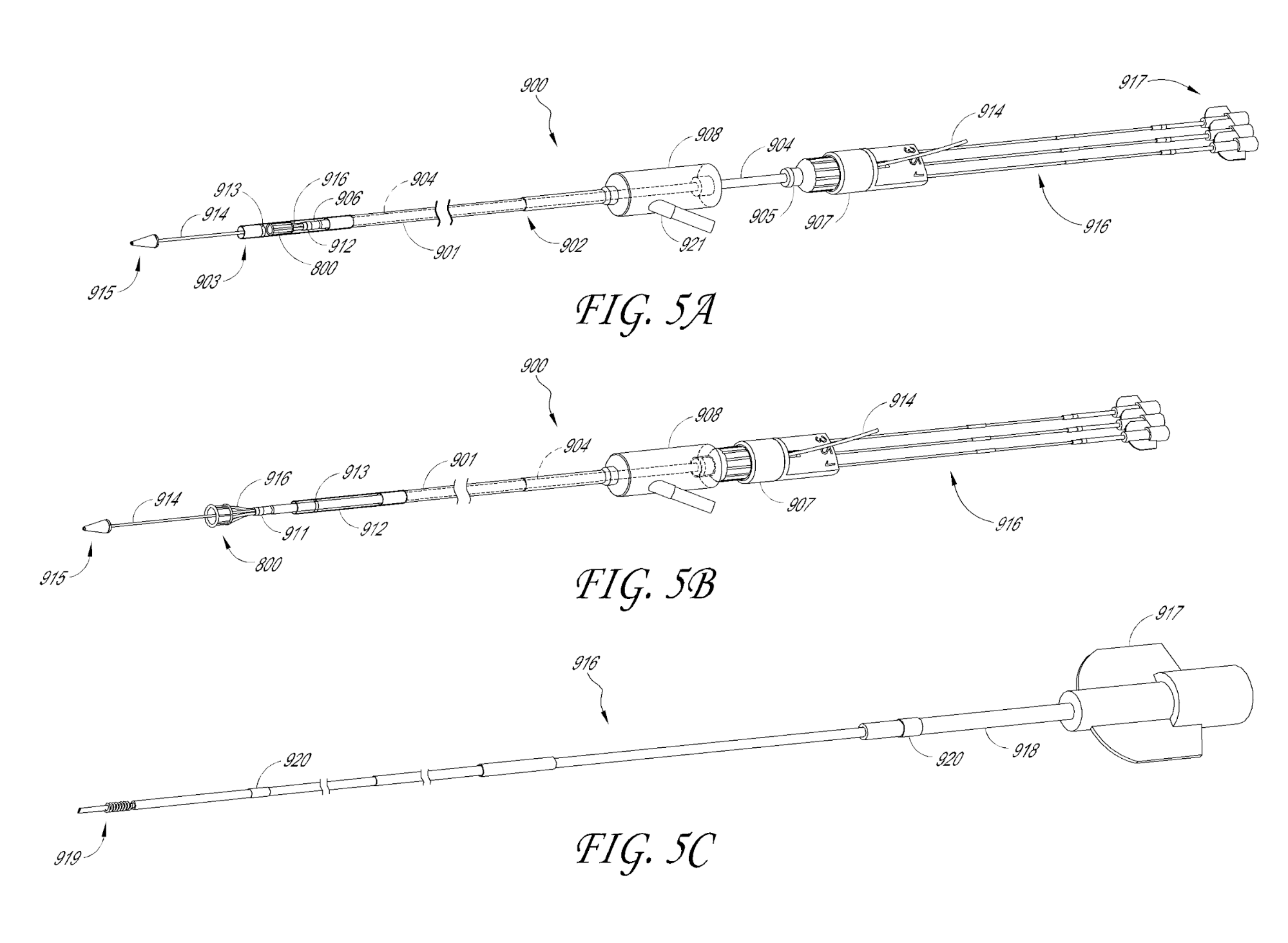
US Patent No. 9,603,708 titled “Low crossing profile delivery catheter for cardiovascular prosthetic implant” granted on March 28, 2017, relates to a minimally invasive delivery method for deploying a cardiovascular prosthetic implant using a delivery catheter having a proximal end and a distal end. The distal end has an outer diameter of 18 French or less and includes a cardiovascular prosthetic implant with an inflatable cuff coupled to a tissue valve.
Contact us for a custom entity analysis report. To know more about MaxVal’s patentability, FTO and validity/invalidity services please click here.